PsiRealm
Quantonix Research • January 2024

Biosensing represents the bleeding edge of healthcare and environmental monitoring technology. The ability to detect the concentration of a specific substance in a biological or chemical reaction has revolutionized the way we diagnose disease, assess environmental pollutants, monitor health conditions and so much more.
Glucose meters, pregnancy tests, and heart rate monitors are all examples of common biosensors that we use in day-to-day life, without even realizing it!
It turns out that light, "squeezed" to reduce its quantum uncertainty, is the key to more sensitive biosensors.
But how do you even "squeeze" light? Certainly not with your fingers!
To begin, let's look at a brief, high-level overview of how biosensors function.
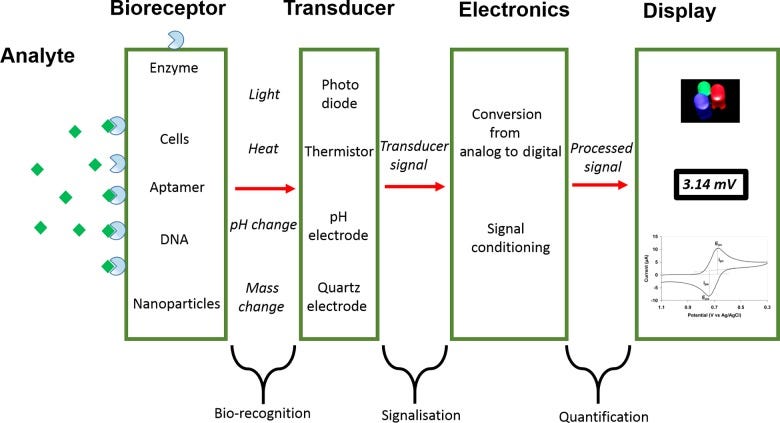
The substance to be detected is called an Analyte. To detect that Analyte, we need to use a molecule referred to as a Biorecepter.
DNA, enzymes, and cells can all function as Biorecepters.
The interaction between the Bioreceptor and the Analyte generates a signal in a process called bio-recognition. However, this signal is quite useless for us humans to understand.
Which is why this signal is then fed into a Transducer. To put it simply, it converts energy from one form to another. In this case, we are using them to convert the bio-recognition signal into something more measurable like an optical or electrical signal.
The exact signal depends on the type of reaction taking place between the Biorecepter and Analyte. Unfortunately, optical or electrical signals are still pretty difficult to understand for us humans.
And so the final step is to process the signal from the transducer by amplifying it, converting it using an ADC (Analog to Digital Converter), and then displaying it on screen.
One of the most common types of Biosensors is called an SPR (Surface Plasmon Resonance) Biosensor. This type of bio-sensor has a wide variety of applications in personalized medicine and environmental monitoring, making them indispensable.
They use Plasmons to help detect changes in how light behaves at a metal sensor surface where biological interactions happen.
Plasmons, specifically Surface Plasmons, are the collective oscillations of free electron gas density at the interface between a metal and a dielectric material, creating a wave that propagates along the surface of the metal.
When light hits the surface of the SPR Biosensor, if analytes are bound to the sensor, they change the amount of light that is bent or slowed down.
This affects the excitation of the Surface Plasmons, causing a shift in the condition of the angle or wavelength where the light is absorbed by the surface plasmons. As a result, there is a measurable change in the angle or intensity of the reflected light, indicating the presence of Analytes.
SPR Biosensors face several challenges that limit their sensitivity:
To understand Squeezed Light and its importance to SPR Biosensors, we first need to understand some basic Quantum Mechanics, and how light acts at the sub-atomic level.
One of the fundamental principles of Quantum Mechanics is the Uncertainty Principle, which states that it is impossible to simultaneously know both the exact position and momentum of a particle.
In fact, the more precisely one property of a particle is measured (either position or momentum), the less precisely the other can be known! As far as we know, this is a fundamental property of nature — Weird and spooky, right?
By reducing quantum noise and minimizing backaction, squeezed light enables the detection of smaller analyte concentrations and more precise measurements of biological interactions.
With continued improvements, you can expect to see an order of magnitude in noise reduction for a practical optical sensor, directly improving SNR.
To extend gains in SNR further, novel detection techniques that make use of squeezing, quantum anticorrelations, and cross-correlation measurements will be required.
While the future looks bright from a technical point of view, no piece of technology is truly world-changing without it being available to the masses, regardless of socioeconomic status.
Let's hope that this technology, and many more to come, will change the lives of everyone who needs it. Now that is a bright future!
ready to
Collaborate?
START HERE